Lander Lab: Cost Efficiency of Baited Ocean Landers

Vessels fitted with a swinging davit arm and winch are ideal for deploying and retrieving Baited Remote Undersea Video (BRUV) systems. In this photo, a Stereo-BRUV system, developed by the Australian Institute of Marine Science (AIMS), is lowered to the seafloor. BRUVs have minimal impact on seafloor communities or the seabed. Photo by Marine Ecology Group - Fish Research, The University of Western Australia
Determining the diversity and distribution of species in an ecosystem is essential to creating a baseline for monitoring studies or to assess the success of conservation and restoration strategies. The methods for sampling marine and aquatic ecosystems can be inefficient and biased, such as diver operated video, or harmful to the environment and biodiversity, such as bottom trawls and seine nets. Both are time-consuming and expensive. Choosing an efficient and cost-effective sampling method to establish baselines and monitor biodiversity is an important consideration in an ecological study.
Two non-invasive techniques for monitoring the sea life in the full water column of a chosen site were considered: baited camera systems, and metabarcoding of environmental DNA (eDNA). Cost comparisons, strengths, weaknesses, and measures of effectiveness are determined.
“While the area of study was localized,” writes Clark in her paper, “the findings presented herein are applicable to global aquatic biodiversity and conservation monitoring programs.”
Baited Cameras
Automatic time lapse cameras for benthic studies have been in use since the 1950’s by researchers such as Prof. John D. Isaacs, Scripps Institution of Oceanography/UCSD, and Dr. Harold E. Edgerton, Woods Hole Oceanographic Institution.
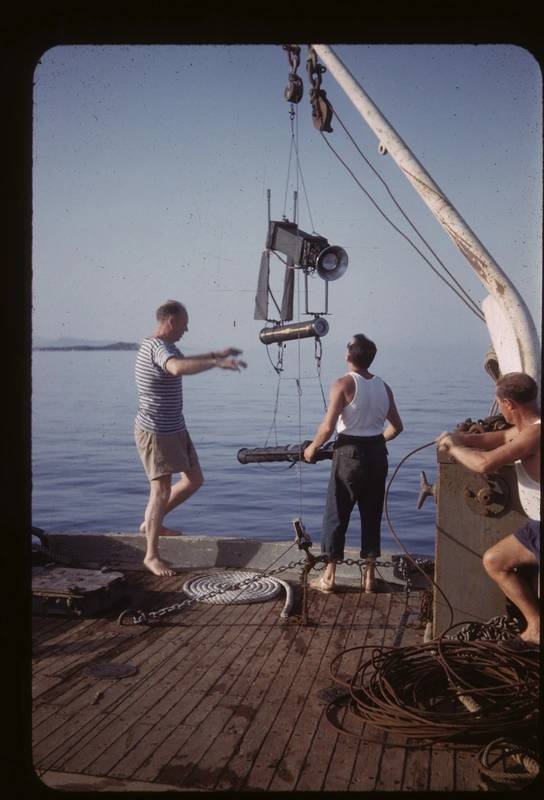 WHOI’S Harold E. Edgerton (left) assists in deploying a tethered deep-sea camera system onboard Jacques-Yves Cousteau’s Research Vessel CALYPSO during Mediterranean fieldwork in 1953. Photo © 2010 MIT. Courtesy of MIT Museum
WHOI’S Harold E. Edgerton (left) assists in deploying a tethered deep-sea camera system onboard Jacques-Yves Cousteau’s Research Vessel CALYPSO during Mediterranean fieldwork in 1953. Photo © 2010 MIT. Courtesy of MIT Museum
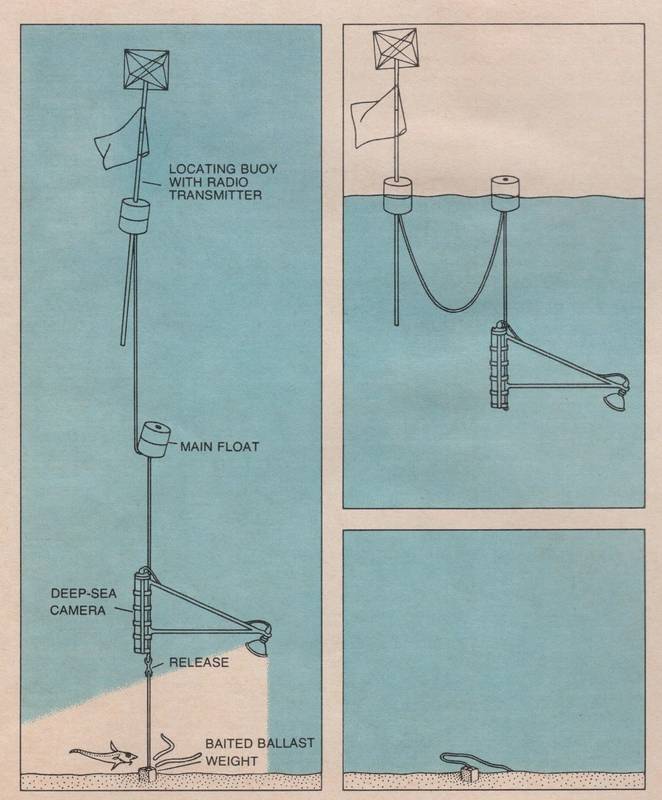 Scripps Institution’s John D. Isaacs’ baited camera free falls into the deep sea to reveal unknown fish species and other scavengers that are attracted to bait attached to the anchor in 1968. Isaacs’ work showed that still images identified sea life, while film clips revealed behaviors. At a pedefined time, the anchor is released and the camera system floats back to the surface where it is recovered. Image courtesy of Scripps Institution of Oceanography/UCSD
Scripps Institution’s John D. Isaacs’ baited camera free falls into the deep sea to reveal unknown fish species and other scavengers that are attracted to bait attached to the anchor in 1968. Isaacs’ work showed that still images identified sea life, while film clips revealed behaviors. At a pedefined time, the anchor is released and the camera system floats back to the surface where it is recovered. Image courtesy of Scripps Institution of Oceanography/UCSD
Baited Remote Underwater Video (BRUV) is an increasingly common, effective, non-invasive and non-destructive method for sampling marine biodiversity. Shallow depths may be explored using a camera system that is lowered to the seafloor like a crab pot and marked with a surface float. Images are often limited to daylight hours, so lights and batteries are not needed. These simple systems are known as “Baited Remote Underwater Video” (BRUV) platforms.
 Stereo Baited Remote Underwater Video (BRUV) at Rheeders' Reef, Tsitsikamma, South Africa. Photo by Peter Southwood, Used with Permission
Stereo Baited Remote Underwater Video (BRUV) at Rheeders' Reef, Tsitsikamma, South Africa. Photo by Peter Southwood, Used with Permission
The use of BRUV systems is favored over extractive sampling methods such as trawling, as many species escape the nets, or are destroyed by capture. Because they are quiet and offer bait, the BRUVs provide a 40% more efficient approach to recording species counts than diver video transects. Additionally, BRUV offers a permanent sampling record that can be reviewed to reduce interobserver variability, provides data on habitat types and can be deployed in deep or heavily structured ecosystems.
BRUVs can provide relative measures of species richness and abundance in a diverse range of conditions and habitats. Stereo-BRUV systems can determine the body size of fish and also produce a digital depth-of-field map, where features and creatures can stand out from their surrounding environment. Fish size can be used as a proxy for biomass, an essential metric for fisheries management reporting. More complex surveys might include a sensor suite to measure and record the effect of fluctuating oxygen minimum zones, and other physical oceanographic events on local populations of animals. Multiple BRUV/ocean lander systems can be deployed at one time, making it a time-efficient survey method of a large area.
Multi-camera digital imaging systems with overlapping fields of view can provide 360° panorama views of the seafloor around a lander. Thermal cameras are finding relevance in marine studies.
As Isaacs showed, deeper waters can be accessed by an autonomous ocean lander in the same manner as a BRUV, relying on the lander’s ability to release a drop weight to float up to the surface. The anchor may be released by a timer or acoustic command. The cost of the anchor and environmental impact can be mitigated by use of ferrocement. (See Marine Technology Reporter, November/December 2024, pp. 40, Lander Lab #12, “Ferrocement Anchors.”)
Like BRUVs, ocean landers provide long duration, quiet, non-invasive platforms to observe marine life, or monitor changing ocean conditions. A second timer can close a small Niskin bottle using a burnwire, capturing a near-bottom water sample for eDNA analysis, as discussed below. The sample of water only adds weight to the vehicle when it is pulled out of the sea and on to the boat.
Other studies have highlighted the limitations of BRUV/Landers. Species ID can be challenging in turbid water environments due to low visibility and there can be overrepresentation of apex predators due to species behavior associated with scavenging. In addition, the scent of the bait will draw species from other areas that may not necessarily be local to the sites sampled, thus the true sampling area is largely unknown. Fish higher in the water column, and cryptic (camouflage) and sedentary species are also likely to be under-represented when using BRUV. Finally, footage analysis can be labor-intensive, time-consuming and costly.
Metabarcoding of environmental DNA (eDNA) is a high-throughput DNA sequencing technique that has become increasingly popular in surveying marine ecosystems. An important advantage of using eDNA is the simplicity of sample collection: a relatively small volume of water, 2L, is needed. Unlike traditional DNA barcoding which identifies a single individual, metabarcoding simultaneously identifies multiple species, providing a broad overview of biodiversity. Genetic material shed by organisms in the ocean such as skin cells, scales, feces, gametes, and other organic material are found in sediments and near bottom (demersal) seawater. These are deposited over a time scale longer than a video clip, and may show species not seen in short duration imaging during daylight hours. Scant DNA can be amplified using polymerase chain reaction (PCR) techniques.
The eDNA method allows for the rapid assessment of diverse ecosystems, the detection of invasive species, and estimates of community composition, painting a portrait of the biodiversity and biomass in a given ecosystem. While DNA will degrade over time, it remains in the environment long enough for the presence of the organisms to be detected without being directly observed or caught. The filtering process is simple, requiring very little training, expertise and time in the field. The technique removes the need for extensive taxonomic expertise to identify species typically required by photographic methods.
Despite the many advantages of eDNA-based monitoring, it also has limitations. Several factors can influence the detectability of eDNA in the environment, leading to either false negatives (failed detection of species present in the area) or false positives (detection of species not present in sampled area). The probability of detection can be influenced by biotic and abiotic factors, including species-specific eDNA generation and degradation, linked to body size, life history stage, diet and migration. The transport of eDNA by high tidal ranges or ocean currents and its rate of decay due to UV strength, pH and water temperature can also affect detection probability. Additionally, contamination of the sample is possible from sample collection through to processing.
Another limitation of eDNA is reference databases used to translate the operational taxonomic units (OTUs) are still incomplete, especially for species found in parts of the world where less research has been carried out.
The Sussex Bay, UK, Study, 2021
The objectives of this study were to: (1) compare species assemblage metrics obtained using BRUV and eDNA; (2) compare the sensitivity of two eDNA metabarcoding primers; (3) investigate the importance of eDNA replication and (4) compare the cost and effort required of both survey techniques for detecting the presence of marine vertebrate species.
Sussex Bay, on the south coast of the UK, was the site selected to test these biomonitoring methods. The presence of marine vertebrate biodiversity was examined at 29 different sites chosen by previous towed video transects. The samples were collected between the 5th and 21st of July 2021, between 8 am and 5 pm.
In their study, a BRUV platform with three cameras was utilized, two looking in one direction, a third looking rearward. The video images were analyzed using footage from the right-hand camera only, using the left-hand camera in case the right camera failed or was obstructed by seaweed. As images were taken during daylight hours, no lights were used.
Three BRUV systems were deployed in succession by boat at each of the 29 sites, 150 m apart and left to film on the seafloor for up to 75 min. Fish and marine vertebrates were observed and identified to the lowest taxonomic level possible.
eDNA samples were collected at each of the 29 sites while the BRUV rigs were deployed. A Kemmerer sampler, triggered by a messenger weight, was used to collect water samples one meter above the seafloor. To minimize eDNA contamination and degradation, each sample was filtered immediately on the boat. In total, 87 BRUV deployments were carried out and 87 eDNA samples were collected in this study.
Results
Used together, eDNA Metabarcoding and video surveys offer strong potential to monitor ocean ecosystems at a variety of depths.
The Sussex Bay studies comparing eDNA and underwater videos found that BRUV surveys cost less overall, but that eDNA monitoring results in better value when accounting for the number of species detected. However, in contrast to BRUV surveys, the species detected by eDNA likely represent the species present within a larger geographical area than the specific site sampled, as noted above.
There may also be an inherent bias due to the time variation in the accumulation of eDNA material versus the time of day and length of the video sample. Also, bottom cameras are unlikely to see fish in the water column overhead.
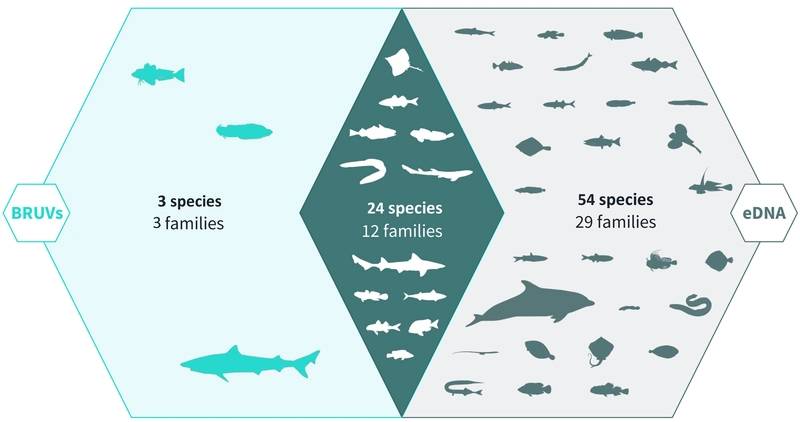 Venn diagram of eDNA and BRUV species detections. Environmental DNA (eDNA) surveys captured the majority (78/81) of the species detected by both surveys, the center and right sections of the Venn diagram. BRUV surveys identified 27/81 species, the center and left sections of the Venn Diagram. Both methods identified the same 24 species belonging to 12 families. Image used with permission. Infographic designed by Alice Clark in collaboration with NatureMetrics
Venn diagram of eDNA and BRUV species detections. Environmental DNA (eDNA) surveys captured the majority (78/81) of the species detected by both surveys, the center and right sections of the Venn diagram. BRUV surveys identified 27/81 species, the center and left sections of the Venn Diagram. Both methods identified the same 24 species belonging to 12 families. Image used with permission. Infographic designed by Alice Clark in collaboration with NatureMetrics
Cost and Effort Comparison
The BRUV costs included building the camera rigs, the bait, the boat hire and the labor cost for video analysis. As the BRUV rigs were built the first year and plan to be reused the following four years, the first year is understandably more expensive than subsequent years.
The eDNA costs covered the Kemmerer sampler, the at-sea filtration system, the boat hire, and the eDNA kits, and the analysis by NatureMetrics. Again, the first year is more expensive than subsequent years as purchased equipment will be reused year-to-year. The cost of carrying out the analysis in-house versus outsourcing the analysis was considered. Given a different level of expertise is required for BRUV video analysis than in-house eDNA analysis, the labor costs of each method were accounted for. The time taken to carry out the fieldwork was accounted for.
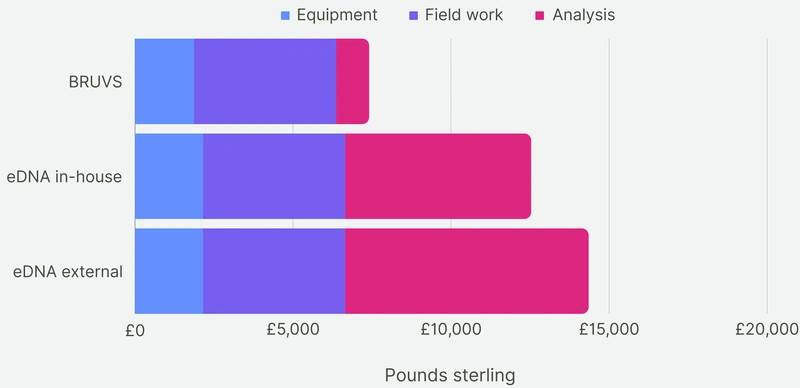 Cost comparison of BRUV surveys, eDNA surveys with in-house sample analysis and eDNA surveys with external sample analysis. Costs were broken down into three categories: equipment, field work and analysis. Overall, the BRUV survey was the most affordable biomonitoring technique, and eDNA with external sample analysis was the most expensive. Infographic was designed by Alice Clark in collaboration with NatureMetrics
Cost comparison of BRUV surveys, eDNA surveys with in-house sample analysis and eDNA surveys with external sample analysis. Costs were broken down into three categories: equipment, field work and analysis. Overall, the BRUV survey was the most affordable biomonitoring technique, and eDNA with external sample analysis was the most expensive. Infographic was designed by Alice Clark in collaboration with NatureMetrics
In the context of a five-year timeframe, the BRUV surveys proved to have the lowest cost compared to both outsourced and in-house eDNA surveys. Nevertheless, eDNA analysis detected a higher species richness, resulting in a lower cost per species detected, with the caveat of earlier described limitations.
When estimating the future costs over five years of sampling the effect of inflation or the likely decrease in cost of eDNA sequencing was not considered.
Equally, video technology is also likely to make great advancements in the coming years. Previous studies estimate the time spent on video footage analysis to be double the length of the recorded video. However, with the rapid development of deep learning and associated AI tools used to automate or partially automate video analysis, the time and effort used for analysis of video footage is likely to be reduced considerably. Camera technology is also becoming more affordable and increasing in resolution quality, allowing more precise identification of population characteristics.
Future Thoughts
A BRUV or ocean lander capable of both video imaging over a minimum 24-hour cycle and water sampling for eDNA, including topside filtering and preservation techniques identified by the Sussex Bay researchers, provides a cost-effective monitoring tool that may be used continuously for several years.
Editor's note:
Operational costs of lander operations are seldom incorporated into scientific articles. This Lander Lab article was inspired by a well-considered academic paper, “Cost-effort analysis of Baited Remote Underwater Video (BRUV) and environmental DNA (eDNA) in monitoring marine ecological communities,” by Alice J. Clark, Research Student, University of Sussex, Brighton, UK, et al. The authors’ estimations of cost effectiveness of two non-invasive sampling methods were tested in the field, a near shore MPA recovering from years of trawling and severe storms. The methods fit the area and time duration of the study. Additional material has been added to this article to supplement the original paper. Readers are encouraged to read the original full text on-line, especially for details of the scientific methods, analysis, and conclusions. Citation below. Dr. Zachary Graff, Research Scientist at the NGO “Beneath the Waves” (beneaththewaves.org) suggested the subject of BRUVs described in this article.
Citations
Clark AJ, Atkinson SR, Scarponi V, Cane T, Geraldi NR, Hendy IW, Shipway JR, Peck M. 2024. Cost-effort analysis of Baited Remote Underwater Video (BRUV) and environmental DNA (eDNA) in monitoring marine ecological communities. PeerJ 12:e17091
Full paper with citations may be found at PeerJ https://peerj.com/articles/17091/
Published April 30, 2024
“Lander Lab” is a hands-on column of Ocean Lander technologies and strategies, a unique class of unmanned undersea vehicles, and the people who make them. It is meant to serve the global ocean lander community in the manner of Make Magazine and other DIY communities.
Comments on this article, or suggestions for stories of interest to other Landereans are welcome. Other ocean lander teams are encouraged to write in about their work. MTR invites you to contact Kevin Hardy <[email protected]>.
















 December 2025
December 2025



